Project
Denitrification control in agricultural soils
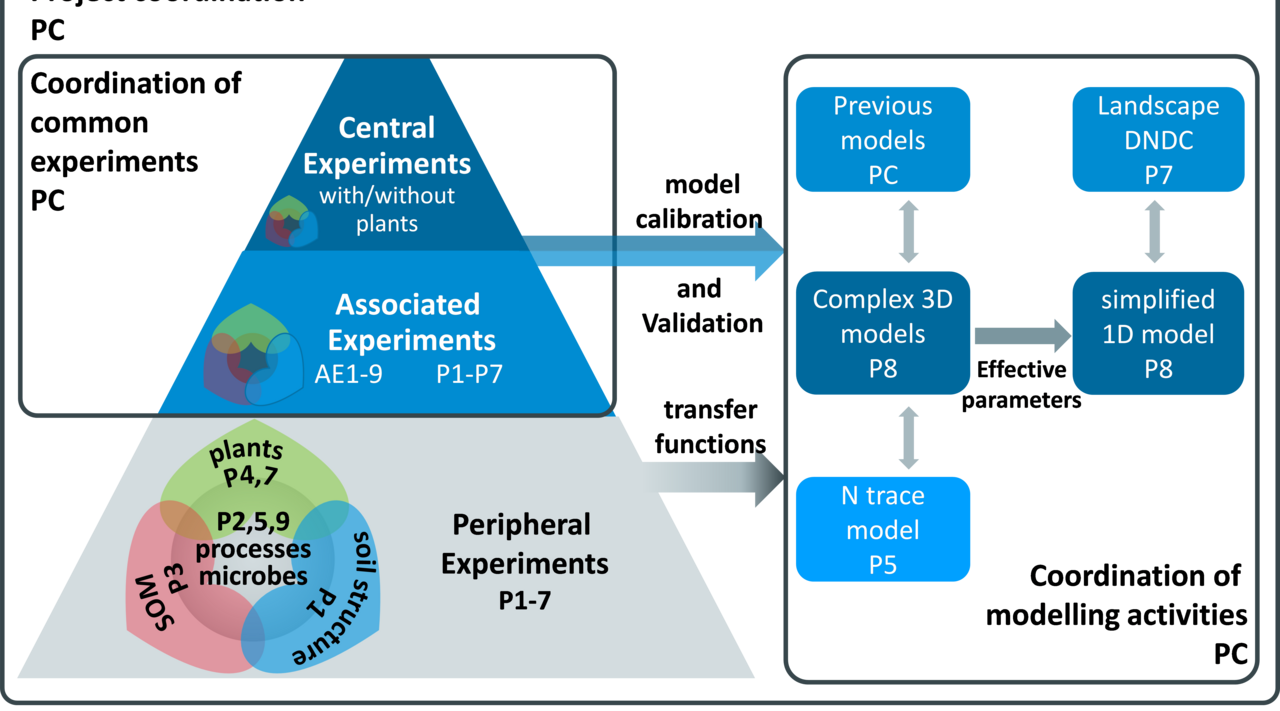
Soil incubations for model validation data sets and experiments to quantify the anaerobic soil volume fraction – Subproject P6 of the research unit "Denitrification in Agricultural Soils: Integrated control and Modelling at various scales” (DASIM) (DFG RU 2337)
To better predict gaseous nitrogen emissions from agricultural soils via microbial processes robust data on N2 emissions and oxygen depletion in soil are needed. Our task is to conduct laboratory studies in order supply these missing data.
Background and Objective
Denitrification is an anaerobic microbial process of successive reduction of nitrate (NO3-) and nitrite (NO2-) to molecular nitrogen (N2) through the following reaction steps: NO3- → NO2- → NO → N2O → N2. A significant fraction of nitrous oxide (N2O) produced in this process is not further reduced to N2 and constitutes the main emission source of this greenhouse gas from agricultural soils. Hence, our understanding and ability to quantify soil denitrification is crucial for mitigating nitrogen fertiliser loss as well as for reducing N2O emission. Robust denitrification data suitable to validate N2 fluxes in denitrification models are scarce due to previous methodical limitations and the extreme spatio-temporal heterogeneity of denitrification. The coordinated DFG research unit „Denitrification in Agricultural Soils: Integrated Control and Modelling at Various Scales (DASIM)” (https://www.thuenen.de/de/ak/aktuelles-und-service/detail-aktuelles/news/detail/News/neue-dfg-forschergruppe-beschaeftigt-sich-mit-der-denitrifikation-in-agrarboeden/) investigates the denitrification process chain in agricultural soils using advanced analytical and molecular biologic methods as well as field studies and various modelling approaches. The aim is to investigate activity and regulation of denitrification in unpreceded spatial and temporal resolution and to use results to develop mathematical models from the micro-scale to the field scale and to improve existing simulation approaches. The anaerobic soil volume fraction, a major control of denitrification, is known to depend on the spatial distributions of gas diffusivity and respiration. However, estimating the anaerobic soil volume fraction has been hampered in the past by the difficulty to quantify its controls. Today, new and improved methods are available to fill those gaps.
The objectives of our subproject P6 are to:
- quantify N2, N2O and NO fluxes and the contribution of different N-transformation processes under variable denitrification control factors (organic C, mineral N, water potential, pore volume) as a basis to validate existing denitrification models.
- provide data sets comprising as much as possible detail on activity and regulation (rates and types of N2 and N2O producing processes as well as the physical, chemical and microbiological control factors) as a basis to calibrate and validate new DASIM models.
- determine the minimum averaging volume for N2 and N2O fluxes from arable soils.
- determine the anaerobic soil volume fraction of DASIM soils under varying conditions.
- visualize organic matter distribution in selected soil samples at the µ-scale.
- test the feasibility to localize denitrification activity in selected soil samples at the µ-scale.
Approach
Our objectives will be tackled in four parts:
- We will use new and improved stable isotope approaches to provide denitrification data sets comprising as much as possible detail on activity and regulation as a basis to validate existing and calibrate new denitrification models that are applied and/or developed by other DASIM subprojects.
- Incubations with increasing soil volumes under standardized conditions will be conducted to determine the minimum elementary volume of denitrification as a basis for upscaling.
- In cooperation with other subprojects we will employ spatial gas diffusivity measurement and modeling as well as denitrification measurements at defined O2 status to obtain independent estimates of the anaerobic soil volume fraction. These approaches will be cross-validated and the most promising one will be used to calibrate the anaerobic soil volume fraction in the new denitrification models.
- We will test to which extent the measurement of the µ-scale distribution of denitrification and its controls is feasible using nano-scale secondary ion mass spectrometry (NanoSIMS) and whether it is a suitable tool to further investigate µ-scale heterogeneity in phase 2 of DASIM. This test includes the measurement of organic matter distribution within selected soil aggregates and a new 15N tracer approach to locate nitrate consumption in aggregates.
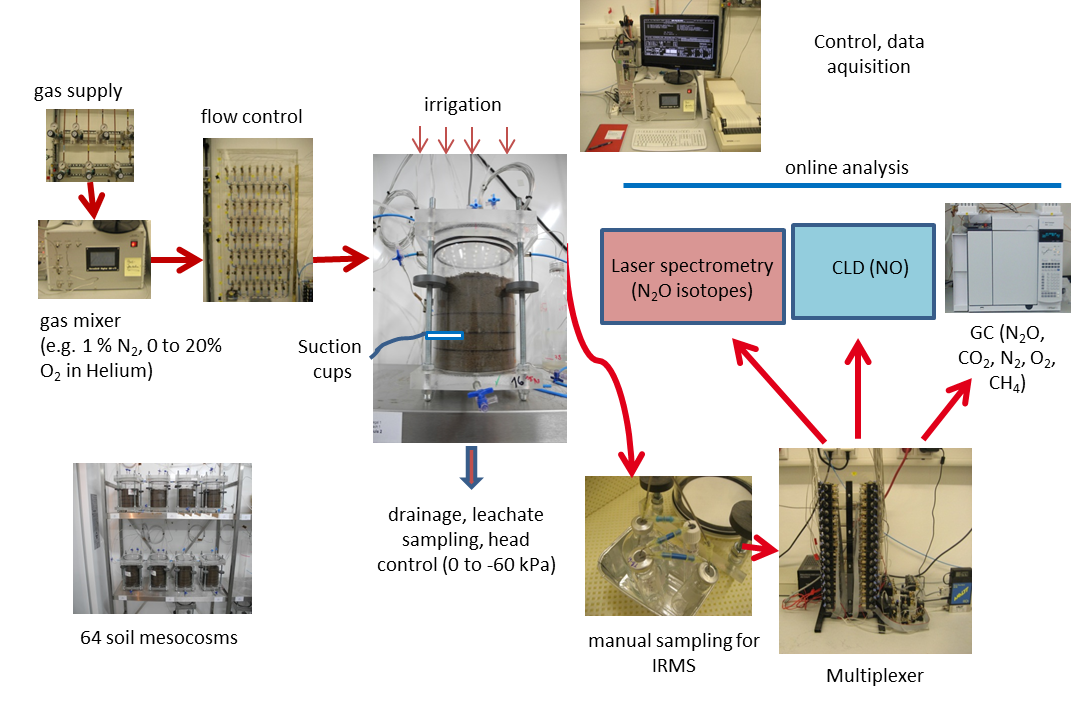
Our Research Questions
The open questions addressed within the entire DASIM RU are:
- How is the activity of denitrifiers and their community structure controlled at the micro-scale?
- How is denitrification affected and controlled by other simultaneously occurring N cycling processes?
- What controls the development of “hot spots” (i.e. specialised habitats) and “hot moments” (i.e. temporary hot spots) for denitrification activity?
- Do fundamental relationships exist between controls of denitrification at various scales (from micro- to meso- and plot-scale)?
- Is it possible to predict denitrification of a given soil in response to atmospheric boundary conditions based on measurable structural and biochemical properties?
The questions addressed in our subproject P6 are:
- How are N2, N2O and NO fluxes and their interaction with different N-transformation processes affected by physical, chemical and microbiological control factors?
- What is the size of the minimum averaging volume for N2 and N2O fluxes from arable soils?
- How can we quantify the anaerobic soil volume fraction and to which extent is the measurement is this quantity suitable to improve denitrification models?
- How is soil organic matter distributed at the micro-scale and to which extent is the measurement is this quantity suitable to improve denitrification models
- Can we localise denitrification activity at the micro-scale using nano-scale secondary ion mass spectrometry (NanoSIMS)?
Results
Thünen-Contact

Involved Thünen-Partners
Involved external Thünen-Partners
-
Technische Universität München
(München, Freising-Weihenstephan, Deutschland) -
Umweltforschungszentrum
(Halle (Saale), Deutschland) -
Leibniz Universität Hannover
(Hannover, Deutschland) -
Martin-Luther-Universität Halle-Wittenberg
(Halle (Saale), Deutschland) - Georg-August-Universität Göttingen
(Göttingen, Deutschland) -
Justus-Liebig-Universität Gießen
(Gießen, Deutschland) -
Universität Rostock
(Rostock, Deutschland) -
Karlsruher Institut für Technologie (KIT)
(Garmisch-Partenkirchen, Deutschland) -
Technical University of Clausthal
(Clausthal, Deutschland) -
Norwegian University of Life Sciences
(Ås, Norwegen)
Funding Body
-
Deutsche Forschungsgemeinschaft (DFG)
(national, öffentlich)
Duration
6.2016 - 5.2019
More Information
Project funding number: FOR 2337
Project status:
finished
Publications
- 0
Rummel PS, Well R, Pausch J, Pfeiffer B, Dittert K (2021) Carbon availability and nitrogen mineralization control denitrification rates and product stoichiometry during initial maize litter decomposition. Appl Sci 11(11):5309, DOI:10.3390/app11115309
- 1
Grosz BP, Well R, Dechow R, Köster JR, Khalil MI, Merl S, Rode A, Ziehmer B, Matson A, He H (2021) Evaluation of denitrification and decomposition from three biogeochemical models using laboratory measurements of N2, N2O and CO2. Biogeosciences 18(20):5681-5697, DOI:10.5194/bg-18-5681-2021
- 2
Lewicka-Szczebak D, Jansen-Willems A, Müller C, Dyckmans J, Well R (2021) Nitrite isotope characteristics and associated soil N transformations. Sci Rep 11:5008, DOI:10.1038/s41598-021-83786-w
- 3
Malghani S, Yoo G-y, Giesemann A, Well R, Kang H (2020) Combined application of organic manure with urea does not alter the dominant biochemical pathway producing N2O from urea treated soil. Biol Fertil Soils 56:331-343, DOI:10.1007/s00374-019-01420-4
- 4
Rummel PS, Pfeiffer B, Pausch J, Well R, Schneider D, Dittert K (2020) Maize root and shoot litter quality controls short-term CO2 and N2O emissions and bacterial community structure of arable soil. Biogeosciences 17:1181-1198, DOI:10.5194/bg-17-1181-2020
- 5
Well R, Buchen C, Köster JR, Lewicka-Szczebak D, Rohe L, Senbayram M, Wu D (2019) A critique of the paper "Estimate of bacterial and fungal N2O production processes after crop residue input and fertilizer application to an agricultural field by 15N isotopomer analysis", by Yamamoto et al. (2017), Soil Biology & Biochemistry 108, 9–16. Soil Biol Biochem 135:450-451, DOI:10.1016/j.soilbio.2018.06.008
- 6
Castellano-Hinojosa A, Loick N, Dixon E, Matthews GP, Lewicka-Szczebak D, Well R, Bol R, Charteris A, Cárdenas LM (2019) Improved isotopic model based on 15N tracing and Rayleightype isotope fractionation for simulating differential sources of N2O emissions in a clay grassland soil. Rapid Comm Mass Spectrometry 33(5):449-460, DOI:10.1002/rcm.8374
- 7
Well R, Burkart S, Giesemann A, Grosz BP, Köster JR, Lewicka-Szczebak D (2019) Improvement of the 15N gas flux method for in situ measurement of soil denitrification and its product stoichiometry. Rapid Comm Mass Spectrometry 33(5):437-448, DOI:10.1002/rcm.8363
- 8
Wu D, Well R, Cárdenas LM, Fuß R, Lewicka-Szczebak D, Köster JR, Brüggemann N, Bol R (2019) Quantifying N2O reduction to N2 during denitrification in soils via isotopic mapping approach: Model evaluation and uncertainty analysis. Environ Res 179(Part A):108806, DOI:10.1016/j.envres.2019.108806
- 9
Well R, Maier M, Lewicka-Szczebak D, Köster JR, Ruoss N (2019) Underestimation of denitrification rates from field application of the 15N gas flux method and its correction by gas diffusion modelling. Biogeosciences 16(10):2233-2246, DOI:10.5194/bg-16-2233-2019
- 10
Senbayram M, Well R, Bol R, Chadwick DR, Jones DL, Wu D (2018) Interaction of straw amendment and soil NO3- content controls fungal denitrification and denitrification product stoichiometry in a sandy soil. Soil Biol Biochem 126:204-212, DOI:10.1016/j.soilbio.2018.09.005
- 11
Wu D, Wei Z, Well R, Shan J, Yan X, Bol R, Senbayram M (2018) Straw amendment with nitrate-N decreased N2O/(N2O+N2) ratio but increased soil N2O emission: A case study of direct soil-born N2 measurements. Soil Biol Biochem 127:301-304, DOI:10.1016/j.soilbio.2018.10.002
- 12
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3-A16, DOI:10.1016/j.soilbio.2018.03.020
- 13
Krause H-M, Thonar C, Eschenbach W, Well R, Mäder P, Behrens S, Kappler A, Gattinger A (2017) Long term farming systems affect soils potential for N2O production and reduction processes under denitrifying conditions. Soil Biol Biochem 114:31-41, DOI:10.1016/j.soilbio.2017.06.025
- 14
Loick N, Dixon ER, Abalos D, Vallejo A, Matthews GP, McGeough KL, Well R, Watson CJ, Laughlin RJ, Cárdenas LM (2016) Denitrification as a source of nitric oxide emissions from incubated soil cores from a UK grassland soil. Soil Biol Biochem 95:1-7, DOI:10.1016/j.soilbio.2015.12.009
- 15
Harter J, Guzman-Bustamente I, Kuehfuss S, Ruser R, Well R, Spott O, Kappler A, Behrens S (2016) Gas entrapment and microbial N2O emissions from a biochar-amended sandy clay loam soil. Sci Rep 6:39574, DOI:10.1038/srep39574
- 16
Well R, Butterbach-Bahl K (2013) Comments on "A test of a field-based 15N-nitrous oxide pool dilution technique to measure gross N2O production in soil" by Yang et al. (2011), Global Change Biology, 17, 3577–3588. Global Change Biol 19:133-135, DOI:10.1111/gcb.12005

![[Translate to English:] [Translate to English:]](/media/_processed_/6/4/csm_titel_CO2Kampagne8_afeea2273e.png)
![[Translate to English:] [Translate to English:]](/media/_processed_/4/1/csm_titel_93px_CO2Kampagne8_9b0f3354d4.png)
![[Translate to English:] Logo des Bundesministerium für Ernährung und Landwirtschaft](/media/allgemein/logos/BMEL_Logo.svg)